Surface Tension :
`=>` We know the fact that liquids assume the shape of the container. But
● Why is it then small drops of mercury form spherical bead instead of spreading on the surface?
● Why do particles of soil at the bottom of river remain separated but they stick together when taken out?
● Why does a liquid rise (or fall) in a thin capillary as soon as the capillary touches the surface of the liquid?
`=>` All these phenomena are caused due to the characteristic property of liquids, called `text(surface tension)`.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
Surface tension of a liquid is defined as the force acting at right angles to the surface along one centimetre length of the surface. Thus, the units of surface tension are dynes per cm.
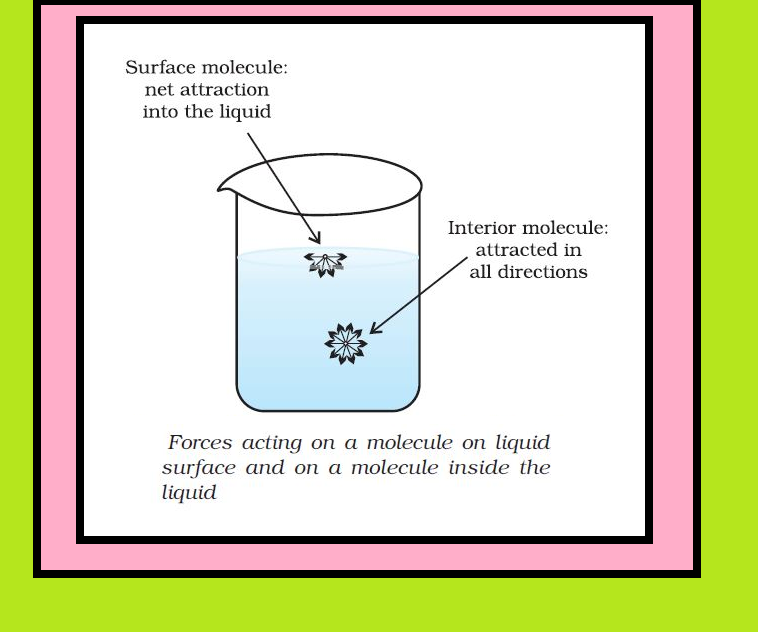
● A molecule in the bulk of liquid experiences equal intermolecular forces from all sides. The molecule, therefore does not experience any net force.
● But for the molecule on the surface of liquid, net attractive force is towards the interior of the liquid, due to the molecules below it. Since there are no molecules above it.
`=>` Liquids tend to minimize their surface area.
● The molecules on the surface experience a net downward force and have more energy than the molecules in the bulk, which do not experience any net force.
● Therefore, liquids tend to have minimum number of molecules at their surface.
● If surface of the liquid is increased by pulling a molecule from the bulk, attractive forces will have to be overcome. This will require expenditure of energy.
`text(Surface Energy :)` The energy required to increase the surface area of the liquid by one unit is defined as `text(surface energy)`.
● Its dimensions are `J m^(–2)`.
`text(Surface Tension :)` It is defined as the force acting per unit length perpendicular to the line drawn on the surface of liquid.
● It is denoted by Greek letter `γ` (Gamma).
● It has dimensions of `kg s^(-2)` and in SI unit it is expressed as `N m^(–1)`.
`=>` The lowest energy state of the liquid will be when surface area is minimum.
● Spherical shape satisfies this condition, that is why mercury drops are spherical in shape.
● This is the reason that sharp glass edges are heated for making them smooth. On heating, the glass melts and the surface of the liquid tends to take the rounded shape at the edges, which makes the edges smooth. This is called `text(fire polishing of glass)`.
● Liquid tends to rise (or fall) in the capillary because of surface tension. Liquids wet the things because they spread across their surfaces as thin film.
● Moist soil grains are pulled together because surface area of thin film of water is reduced.
● It is surface tension which gives stretching property to the surface of a liquid.
● On flat surface, droplets are slightly flattened by the effect of gravity; but in the gravity free environments drops are perfectly spherical.
`=>` The magnitude of surface tension of a liquid depends on the attractive forces between the molecules.
● When the attractive forces are large, the surface tension is large.
● Increase in temperature increases the kinetic energy of the molecules and effectiveness of intermolecular attraction decreases, so surface tension decreases as the temperature is raised.
● Why is it then small drops of mercury form spherical bead instead of spreading on the surface?
● Why do particles of soil at the bottom of river remain separated but they stick together when taken out?
● Why does a liquid rise (or fall) in a thin capillary as soon as the capillary touches the surface of the liquid?
`=>` All these phenomena are caused due to the characteristic property of liquids, called `text(surface tension)`.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
Surface tension of a liquid is defined as the force acting at right angles to the surface along one centimetre length of the surface. Thus, the units of surface tension are dynes per cm.
● A molecule in the bulk of liquid experiences equal intermolecular forces from all sides. The molecule, therefore does not experience any net force.
● But for the molecule on the surface of liquid, net attractive force is towards the interior of the liquid, due to the molecules below it. Since there are no molecules above it.
`=>` Liquids tend to minimize their surface area.
● The molecules on the surface experience a net downward force and have more energy than the molecules in the bulk, which do not experience any net force.
● Therefore, liquids tend to have minimum number of molecules at their surface.
● If surface of the liquid is increased by pulling a molecule from the bulk, attractive forces will have to be overcome. This will require expenditure of energy.
`text(Surface Energy :)` The energy required to increase the surface area of the liquid by one unit is defined as `text(surface energy)`.
● Its dimensions are `J m^(–2)`.
`text(Surface Tension :)` It is defined as the force acting per unit length perpendicular to the line drawn on the surface of liquid.
● It is denoted by Greek letter `γ` (Gamma).
● It has dimensions of `kg s^(-2)` and in SI unit it is expressed as `N m^(–1)`.
`=>` The lowest energy state of the liquid will be when surface area is minimum.
● Spherical shape satisfies this condition, that is why mercury drops are spherical in shape.
● This is the reason that sharp glass edges are heated for making them smooth. On heating, the glass melts and the surface of the liquid tends to take the rounded shape at the edges, which makes the edges smooth. This is called `text(fire polishing of glass)`.
● Liquid tends to rise (or fall) in the capillary because of surface tension. Liquids wet the things because they spread across their surfaces as thin film.
● Moist soil grains are pulled together because surface area of thin film of water is reduced.
● It is surface tension which gives stretching property to the surface of a liquid.
● On flat surface, droplets are slightly flattened by the effect of gravity; but in the gravity free environments drops are perfectly spherical.
`=>` The magnitude of surface tension of a liquid depends on the attractive forces between the molecules.
● When the attractive forces are large, the surface tension is large.
● Increase in temperature increases the kinetic energy of the molecules and effectiveness of intermolecular attraction decreases, so surface tension decreases as the temperature is raised.